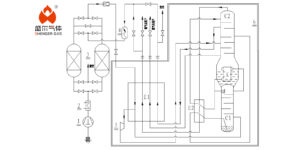
Cryogenic distillation of air, a cornerstone in the industrial gas sector, plays a pivotal role in separating atmospheric air into its primary components: nitrogen, oxygen, and argon. This article delves into the essence of cryogenic distillation, its operational mechanics, its industrial applications, and a comparative analysis with Pressure Swing Adsorption (PSA) technology, highlighting their respective advantages and disadvantages.

What is Cryogenic Distillation of Air?
Cryogenic distillation is a process utilized to separate gases from a mixture at extremely low temperatures. By cooling air to below its liquefaction point, it enables the efficient separation of its components based on their boiling points. This method is highly favored for its ability to produce high-purity gases on a large scale.
How Cryogenic Distillation Works
The cryogenic distillation process begins with the purification of air to remove impurities such as water vapor, carbon dioxide, and hydrocarbons. Following purification, the air is compressed and cooled through a series of heat exchangers. Upon reaching cryogenic temperatures (below -150°C), the air liquefies.
In the distillation column, the liquid air is gradually warmed, causing different components to vaporize at different temperatures. Oxygen boils at -183°C, nitrogen at -196°C, and argon at -186°C, allowing for their sequential separation. The resulting gases are then extracted and further purified as necessary.
Industrial Applications of Cryogenic Distillation
Cryogenic distillation serves numerous sectors, including:
- Medical Industry: Producing high-purity oxygen for hospitals and healthcare facilities.
- Chemical Manufacturing: Supplying nitrogen and oxygen for various chemical synthesis processes.
- Steel Manufacturing: Providing oxygen for metal cutting and refining processes.
- Electronics: Producing ultra-high-purity gases for semiconductor and LCD manufacturing.
Comparison with PSA Technology
While cryogenic distillation excels in producing high-purity gases on a large scale, Pressure Swing Adsorption (PSA) offers a different approach. PSA operates at near-ambient temperatures and pressures, using adsorbent materials to separate gases based on molecular size and polarity.
Advantages of Cryogenic Distillation:
High Purity: Capable of producing gases with very high purity levels, suitable for critical applications.
Large Scale Production: Efficient for large volume gas production, making it cost-effective for industrial needs.
Disadvantages of Cryogenic Distillation:
High Energy Consumption: Requires significant energy for cooling air to cryogenic temperatures.
Capital Intensive: High initial investment in infrastructure and equipment.
Advantages of PSA:
Lower Energy Requirements: Operates at ambient temperatures, reducing energy consumption.
Flexibility: Easily scalable and adaptable to different production volumes.
Disadvantages of PSA:
Purity Limitations: While PSA can produce high-purity gases, it may not reach the ultra-high purity levels achievable with cryogenic distillation.
Selective Applications: More suitable for specific gases and applications due to the nature of the adsorbents.
Cryogenic distillation and PSA technology each have their unique strengths and limitations, catering to different industrial requirements. The choice between these technologies depends on the specific needs regarding purity, volume, and economic considerations. As the air separation industry continues to evolve, both methods will remain vital in meeting the diverse demands of global markets, driving innovation and efficiency in gas production and applications.