Cryogenic air separation
Cryogenic air separation is a process where atmospheric air is cooled and liquefied to separate its components, primarily oxygen, nitrogen, and argon, based on their differing boiling points. This technology is essential in various industries for producing high-purity gases used in medical, welding, and industrial applications.
purity
99.999%
purity
99.6%
Compression
Ambient air is compressed, reducing its volume and preparing it for cooling. This step increases efficiency for the subsequent cooling process.
Pre-Cooling
The compressed air is pre-cooled using refrigerants, lowering its temperature to just above its dew point, which aids in effective purification.
Purification
Impurities like water vapor, carbon dioxide, and hydrocarbons are removed using adsorbent materials, ensuring clean, dry air for distillation.
Expansion
The cooled air is expanded, reducing its temperature further to enable liquefaction, often using expansion valves or turbo-expanders.
Heat Exchange
The air is further cooled in counter-flow heat exchangers, approaching cryogenic temperatures necessary for liquefaction and separation.
Distillation
In the fractional distillation column, the liquefied air is separated into oxygen, nitrogen, and argon based on their different boiling points, achieving the desired purity levels.
Shenger provides comprehensive services for the design, construction, commissioning, and maintenance of low-temperature stations. The use of the best components and advanced technology ensures the highest reliability and efficiency of the power station.
Cryogenic air separation Generators
Advanced control system
Rapid production of nitrogen
Gas quality is stable
Adsorbent has long service life
High efficiency production
Accelerate regeneration
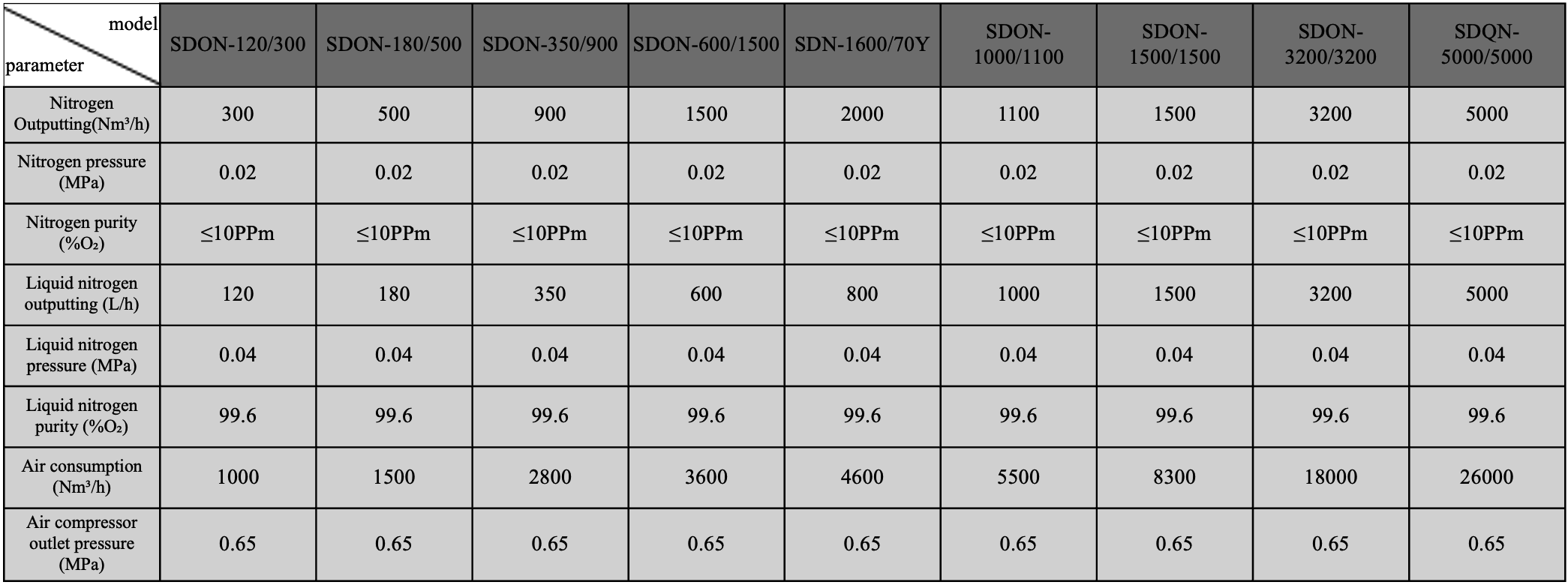
Cryogenic air separation parameter
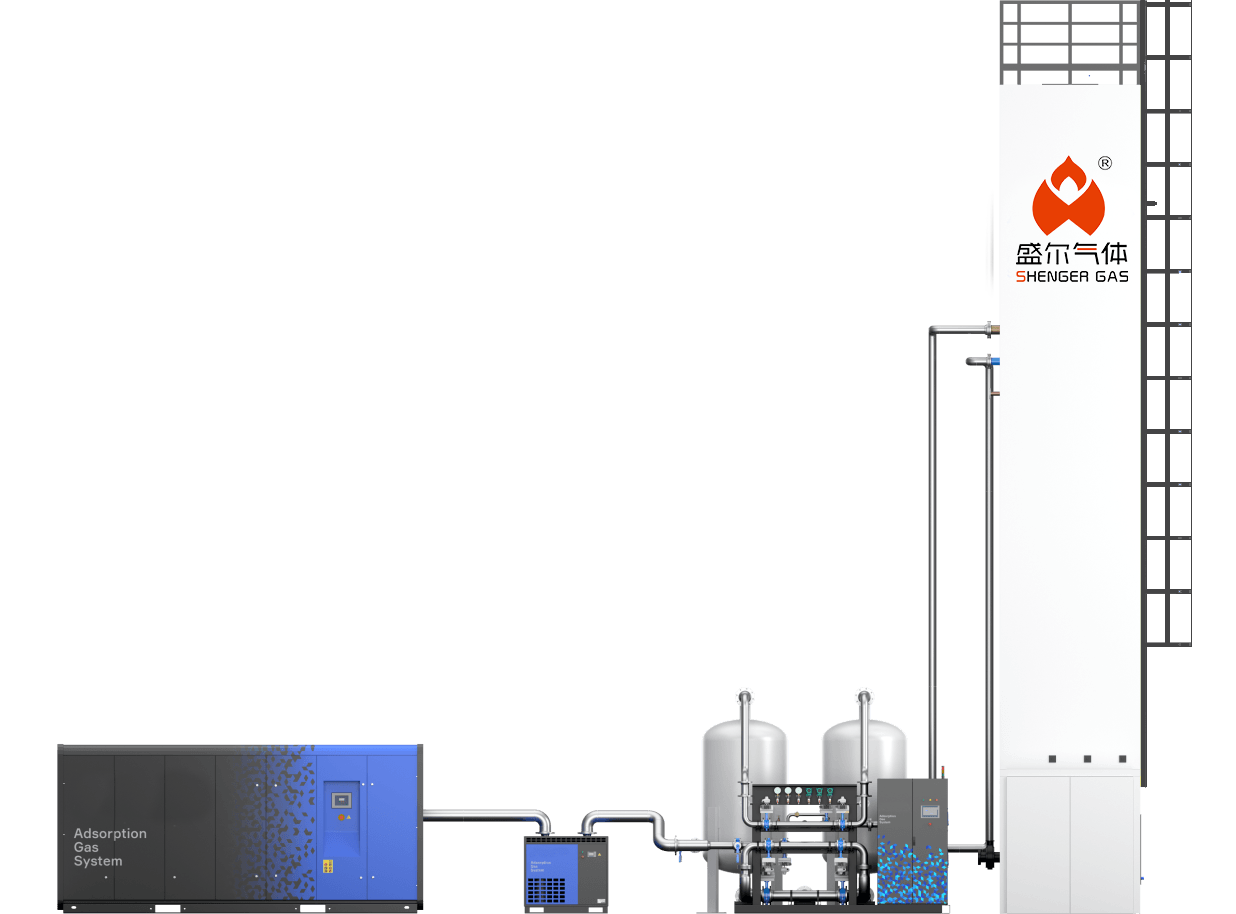
Cryogenic air separation system
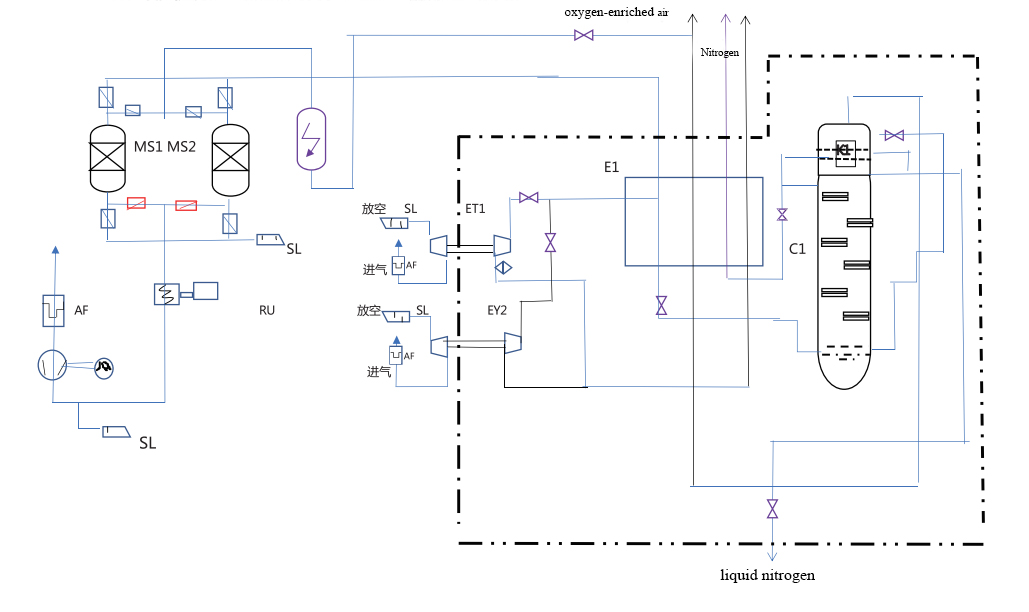
In this initial stage, ambient air is drawn into the system and compressed to a higher pressure. The compression is crucial for increasing the efficiency of the subsequent cooling process. It typically involves multi-stage compressors, where the air is progressively compressed, reducing the volume and preparing it for the next phase of pre-cooling.
After compression, the air undergoes pre-cooling to lower its temperature before further purification. This step is essential to reduce the energy required for the subsequent cooling and liquefaction. Pre-cooling often uses chilled water or refrigerants to bring the air to a temperature just above its dew point, which helps in the effective removal of water vapor and carbon dioxide in the next stage.
Purification is vital to remove impurities like water vapor, carbon dioxide, and hydrocarbons, which can freeze and block the system at lower temperatures. This is typically achieved using adsorbent materials in molecular sieves. The purification stage ensures that only clean, dry air enters the heat exchanger and distillation units, preventing operational issues and maintaining product purity.
In the heat exchange stage, the air is further cooled to approach cryogenic temperatures, essential for its liquefaction. This process is performed in counter-flow heat exchangers, where the incoming air is cooled by the outgoing cold waste gases, improving energy efficiency. The heat exchange stage is critical for reaching the low temperatures required for effective air separation.
The cooled air is then expanded, which reduces its temperature further to the point of liquefaction. This is often accomplished using expansion valves or turbo-expanders. The expansion stage is crucial for lowering the temperature sufficiently to enable the separation of air into its components in the distillation column.
Finally, the liquefied air enters a fractional distillation column, where it is separated into oxygen, nitrogen, and argon based on their different boiling points. This step takes place in a series of distillation trays, with oxygen typically collected at the bottom, nitrogen at the top, and argon extracted from an intermediate level. This stage is key to achieving the desired purity levels of the separated gases.

